Entegris is delighted to announce our new interactive tool intended to help the life sciences industry better understand how we can support the entire drug manufacturing process. Our growing life sciences portfolio can be overwhelming for customers, and this simple tool helps guide them to finding all the ways we can help. The tool was designed to be versatile and can be shared by our team as part of a sales overview, or you can explore it yourself to quickly learn more about the solutions we offer.

“We first launched the interactive tool at the tradeshow Interphex this year and
had tremendous feedback on its ease of use in demonstrating exactly what the customer needs to know about us and how we provide solutions for their specific needs. This is another tool that Entegris offers to help improve customers’ research time in finding products to work within their process. In addition, it sparked many conversations with customers and drove them to the booth.”
- Todd Kapp, Director-Life Science, Global Commercial Development, Entegris
Using the New Life Sciences Interactive Tool
Upon entering our interactive tool, you’ll be able to choose between the Bioprocessing Workflow and the Product Library. The Bioprocessing Workflow shows you the entire bioprocess unit operation workflow with our upstream, downstream, fill/finish, and oligonucleotide application solutions. This lets you quickly focus on the most relevant parts (Figure 1). Here, you can take a virtual walkthrough of cell culture, cell growth, and cell separation in our upstream workflow, and get to know all our downstream offerings from clarification, quality control lab, and bulk chemical to sterile filtration and bulk fill.
Clicking on cell culture in the workflow will take you to all our products in this space. These include bioreactors, 2D and 3D bags, single-use tube sets, tubing and pipe products (Figure 2). You can further investigate our capabilities within each step throughout the entire workflow. In addition, you can add all the appropriate literature, webinars, and white papers to an email to send to yourself and reach out directly to ask further questions or schedule a tour of our Life Sciences Technology Center.
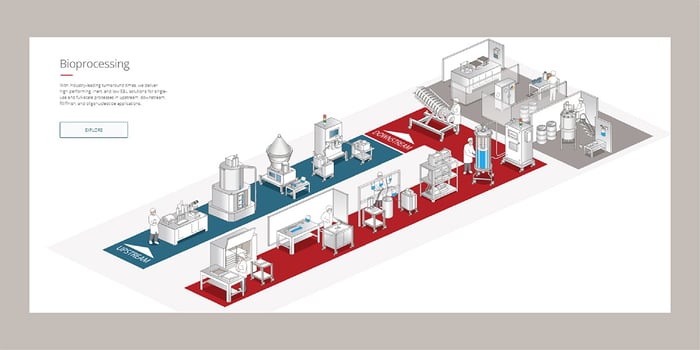 Figure 1. Bioprocess Manufacturing Workflow
Figure 1. Bioprocess Manufacturing Workflow
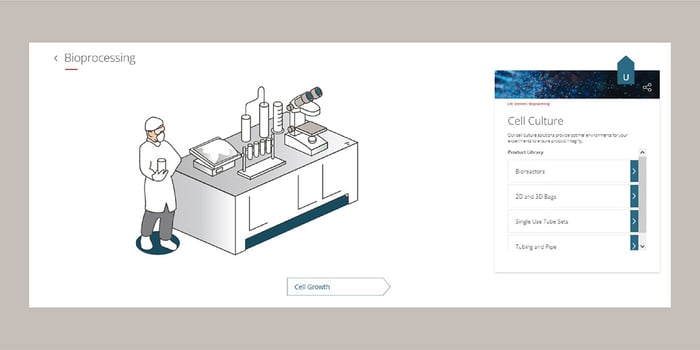 Figure 2. Cell Culture Capabilities
Figure 2. Cell Culture Capabilities
Browsing the Life Sciences Product Library
Already know what products you need? Head directly to our product library, where all our offerings are organized within the following categories: single-use assemblies, filtration and purification, chemical containers, and facility fluid management with easy access to all the relevant documentation (Figure 3).
Through our 50+ years of proven materials science experience, we can easily become your strategic partner by applying our expertise to develop the cleanest, most scalable, and most reliable solutions to reduce your validation time, development costs, and time to market.
 Figure 3. Product Library
Figure 3. Product Library
Check it out for yourself and find out more how we can be your partner in life sciences manufacturing.




