The pharmaceutical and biopharmaceutical industries continue to embrace the utilization of single-use systems (SUS). With the ever-growing adoption of SUS products, increasing scrutiny has been placed on the purity concerns of single-use components and their possible impact on the biomanufacturing, storage and transportation of high value final products.
Aramus™ single-use bag assemblies are fabricated using a single-layer, high-grade, gamma-stable fluoropolymer and offer high purity, exceptionally low extractable and leachable (E&I) profile, excellent chemical compatibility and enhanced safety for crucial process fluids and final products.
Particular attention is given to minimizing particulate contamination using ISO Class 5 cleanroom manufacturing and quality control (QC) testing with the AccuSizer® liquid particle counter.
Reasons particulate levels give cause for concern with include:1
- Cell growth interference
- Drug product quality, toxicity and safety
- Processing interference
- Risk to the patient from possible micro blood vessel obstruction
For these reasons, Aramus bags are fabricated to the highest standard of cleanliness and carefully tested for particulate contamination. As Entegris produces both the Aramus fluoropolymer bag assemblies and the AccuSizer liquid particle counters used for QC testing, they are specially qualified to supply single-use systems that significantly reduce particulate contamination.
Subvisible particulate Aramus bag testing is predicated on the USP standard2 and is authenticated in other Entegris application notes.3,4 These tests highlight the USP limits of less than 25 particles/mL ≥10 µm and less than 3 particles/mL ≥25 µm. One hundred percent of all Aramus bags tested comply with the USP <788> standard.
Adsorption study of mRNA encapsulated lipid nanoparticles
Entegris worked in collaboration with Precision Nanosystems, Inc. (PNI)5 to conduct a study utilizing model mRNA-encapsulated lipid nanoparticles (LNPs) to analyze material adsorption of mRNA-encapsulated LNP over a seven-day storage time, comparing the Aramus fluoropolymer bag to frequently used glass vials and polypropylene (PP) cryotubes.
A solution containing mRNA-encapsulated LNPs was assessed for physicochemical properties after incubation under various conditions to determine initial data on mRNALNPs stability when in contact with these three enclosure materials.
Model mRNA (EPO) were developed and encapsulated into LNPs utilizing PNI’s proprietary lipid mix and formulation system.
They are aliquoted into the enclosures and kept at room temperature, and then evaluated at different time points; at time zero (T0) immediately after formulation, after 24 hours and after 168 hours storage for mRNA-LNP concentration (Figure 1), mRNA concentration (Figure 2) and mRNA-LNP mean size (Figure 3).
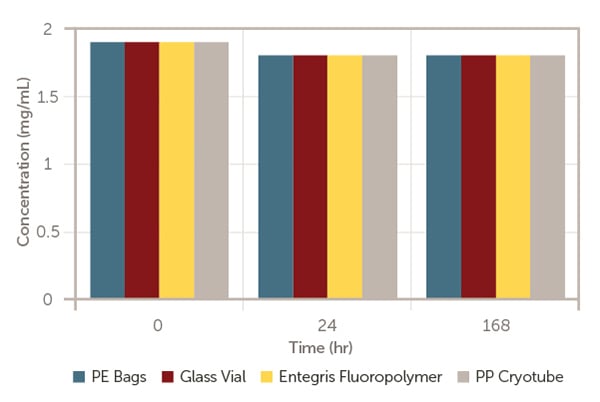 Figure 1. LNP concentration versus time.
Figure 1. LNP concentration versus time.
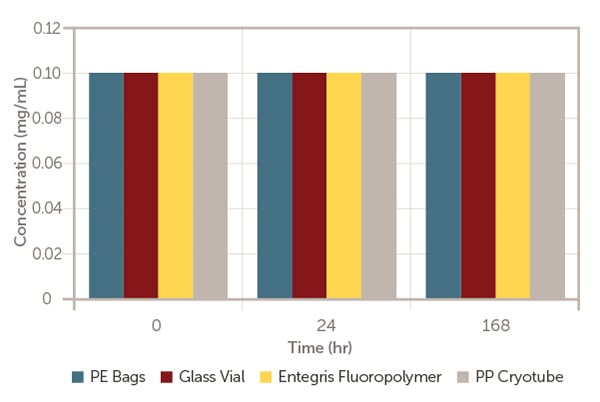 Figure 2. mRNA concentration versus time.
Figure 2. mRNA concentration versus time.
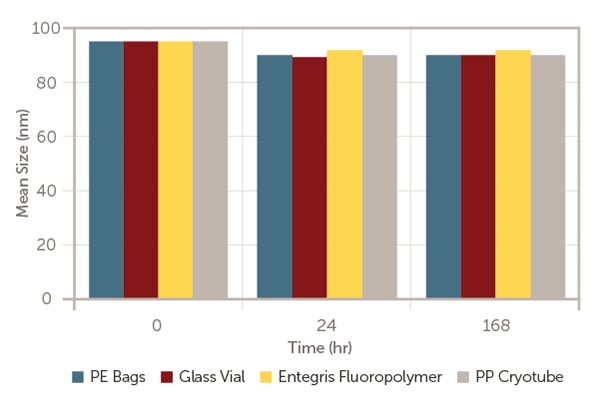
Figure 3. mRNA-LNP mean size versus time.
As exhibited in Figures 1 to 3, after 168 hours of storage at room temperature, model mRNA-encapsulated LNP has retained almost the same amount of lipid nanoparticle concentration, mean size, mRNA concentration and encapsulation efficiency in line with the industrial standard containers; glass vial, PP cryotube, and PE bags.
This indicates that Aramus fluoropolymer bags are appropriate for pharmaceutical use.
The enclosures holding the LNP suspensions were then transported to the Entegris Goleta, CA facility for further assessment utilizing both the Nicomp® DLS and AccuSizer SPOS systems. The Nicomp system data demonstrated an increase in the LNP size from 96 to 134 nm, a probable result of aggregation with time.
The contents of the bags were then evaluated using the AccuSizer SIS system to measure particle size and concentration from 0.5–400 µm. The results displayed in Figure 4 plot particle counts/mL against size for the Aramus fluoropolymer bags versus the polyethylene (PE) bags at T0 and after 168 hours.
The results in Figure 4 signify a considerably greater amount of large particles in the PE bag at both T0 and after 168 hours. This potentially shows a higher degree of LNP aggregation as opposed to more contamination in the PE bags, but this cannot be verified because SPOS is not specific to particle chemistry.
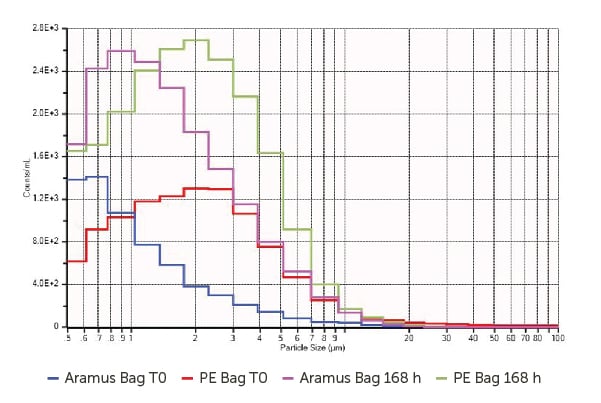
Figure 4. Particle concentration/mL in different single-use enclosures.
However, the size distribution exhibited here shows greater consistency with the inherent aggregated proteins rather than extrinsic contamination.6
While investigating these results, Entegris made the decision to assess particulate contamination in Aramus bag assemblies against other SU bag assemblies in the submicron region.
The drive to reach the highest standard of cleanliness with respect to particulate contamination could incorporate measurements of sizes below 10 and 25 µm.
Bag cleanliness comparison testing
Several SUS bags that were commercially available were acquired, and a particulate contamination comparison study was conducted.
Procedure:
-
Milli-Q water tested to determine background baseline
-
250 mL bag assemblies filled with the Milli-Q water at a surface area-to-volume ratio of SA/V = 6 cm2 /mL (following BPOG guidance)
-
Orbital shaker set at 40 RPM for 2 minutes for agitation of the bag
-
Transfer water from bag to clean beaker
-
Run liquid particle counting test for the water in the beaker
AccuSizer SPOS system protocol:
- Sensor mode = summation
- Sample volume = 5 mL
- Number of replicates = 4
- Flow rate = 30 mL/minute
A comparative analysis of the Aramus bag versus one type of EVA bag from brand A
is displayed in Figure 5.
Table 1. Source: Entegris
| Size | EVA bag | Aramus bag |
| 0.5 − 400 μm | 6871.3 | 1455.3 |
| 1 − 400 μm | 1222.3 | 488.3 |
| 2 − 400 μm | 167.3 | 109.7 |
| 5 − 400 μm | 42 | 22 |
| 10 − 400 μm | 8.7 | 1.7 |
| 25 − 400 μm | 0.3 | 0.3 |
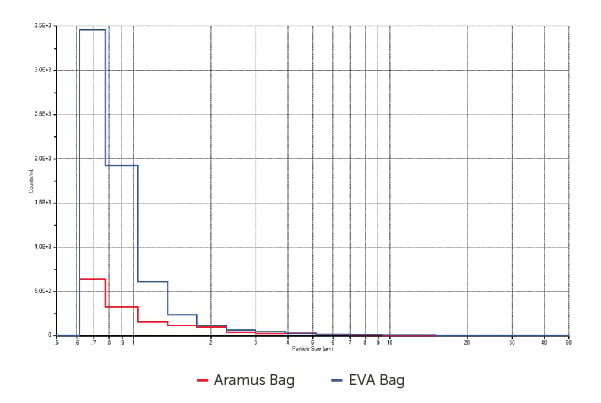
Figure 5. Particle concentration/mL, Aramus bag versus EVA bag
These results signify reduced particulate contamination levels in the Aramus fluoropolymer bags than with the EVA material bags. After studying this data, further tests were conducted in the Entegris Billerica, MA facility assessing the Aramus fluoropolymer against two EVA type bags.
The Billerica, MA studies used the same procedure and protocol as detailed above. As exhibited in Figure 6, two types of 250 mL EVA bag assemblies were contrasted against 250 mL Aramus bag assemblies for cleanliness level.
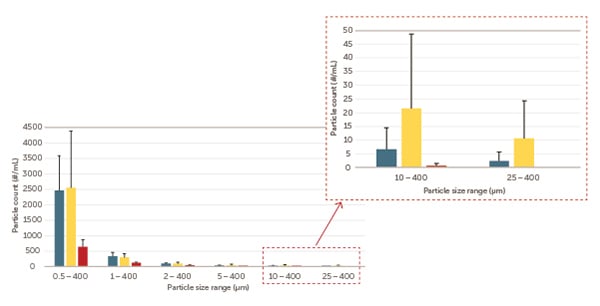
Figure 6. Entegris (red), Brand B (blue), Brand C (yellow) particles concentration/mL versus size (μm).
Aramus bag assemblies systematically met the USP standard for less than three counts of particles/mL larger than 25 µm and less than 25 counts of particles larger than 10 µm, while the two types of EVA bags occasionally surpassed the limits set by USP <788>.
The results displayed in Figure 7 also indicate that the particulate contamination of the Aramus bag assemblies was at the lowest level at >0.5 µm range, which is around half of that of brand B and a third of brand C.
Aramus bag assemblies also exhibited a much more consistent level of cleanliness from assembly to assembly when compared to EVA brands B and C.
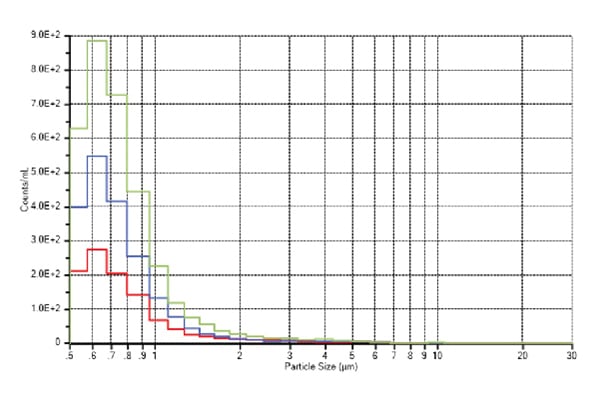
Figure 7. Entegris (red), Brand B (blue), Brand C (green) particles concentration/mL versus size (μm).
Conclusions
This study indicates that Aramus fluoropolymer bag assemblies set a higher standard of cleanliness than bags from competitors. Aramus bag assemblies routinely meet the USP standard, while at times, competitors’ cryobags fall outside of the standard.
Aramus bag assemblies can deliver a more consistent performance in between different bags, providing customers with quality assurance. It was also observed that Aramus bag assemblies are two to three times cleaner in the submicron particle range when compared with the EVA bags of its competitors.
This implies that Aramus bag assemblies can be an excellent single-use system for biopharmaceutical industry applications.
For more information, view our on-demand webinar regarding this topic: Particulate Contamination in Medical Devices and Single-Use Systems
References
- Recommendations for Testing, Evaluation, and Control of Particulates from Single-Use Process Equipment, BPSA 2014 report
- USP, Particulate Matter in Injections
- Entegris Application Note, Aramus Single-Use Bag Particle Testing, November 2018
- Entegris Application Note, Monitoring Particulate Contamination in Medical Devices, March 2021
- Precision Nanosystems, Inc., www.precisionnanosystems.com
- Chou, K. and Bumiller, M., Opportunities and Pitfalls in the Analysis of Subvisible Particles during Biologics Product Development and Quality Control, American Pharmaceutical Review, August 2020





