Overview
The rapid increase of semiconductors in cars enables significant safety, connectivity, mobility, and sustainability improvements. As transportation transforms from being driver controlled to software controlled, automakers must look closer at their ability to measure and maintain product reliability throughout the vehicle’s lifetime.
In today’s microelectronics manufacturing facilities, Advanced Fault Detection Classification (FDC) is required even more to guarantee quality in terms of process stability, defect reduction, and tighter uniformity.
In collaboration with STMicroelectronics, this case study looks at how to effectively collect data to improve your process control strategy and lower defectivity.
Customer Issue
Our customer’s resist dispense pump installed base was old and aging. Filtration was not always under control and thus negatively affecting dispense repeatability and stability. In addition, they were looking for a way to collect detailed dispense line status data to improve their photolithography process.
Approach 
We proposed they evaluate our IntelliGen® LV two-stage technology dispense system that offers:
1. independent filtration and dispense functionality
2. dispense monitoring and confirmation capabilities
Two-Stage Dispense Technology
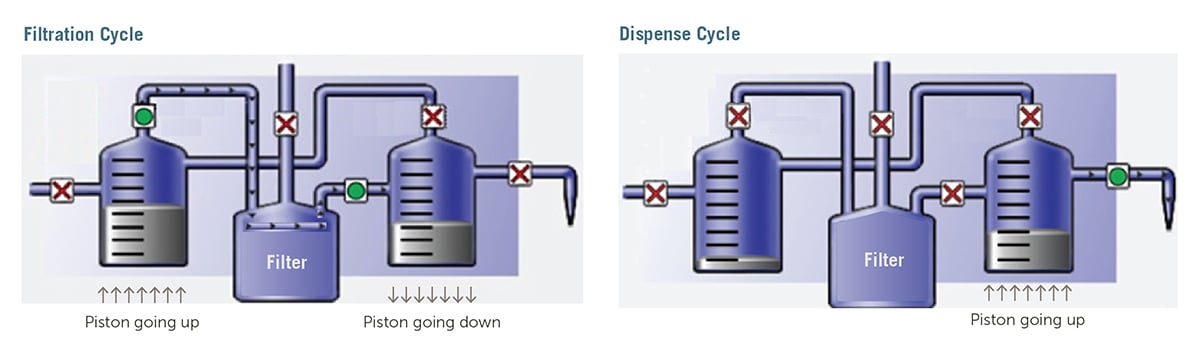
| Figure 1: During filtration (idle times between dispenses), the dispense pump's motor reverses direction and the feed pump pushes the fluid slowly through the filter at the programmed rate (typically 0.1 to 0.5 mL/sec). |
Figure 2: During dispense, the filter is isolated from the dispense chamber to minimize pressure changes that affect repeatability. Typical dispense rates are between 1 and 2 mL/sec. |
Read the full case study to better understand the difference with a single-stage pump technology and know more about the dispense monitoring and confirmation capabilities.
Results
Thanks to Entegris’ solution, STMicroelectronics achieved
- Three times lower wafer defectivity
- 30% tighter wafer dispense uniformity
- Stability over six months of operation
Download the full case study to see the experiment design and illustrations of the results.
Conclusion
According to STMicroelectronics, the IntelliGen dispense pump from Entegris is one of the best pump resist systems. It enabled guaranteed process stability, thickness uniformity, defectivity reduction, and statistical process control (SPC.) The network communication capability allowed data collection and storage in fab management systems. All these features combined to greatly improve the process quality and significantly reduce wafer excursions.
For more information on this solution, please visit the IntelliGen LV Dispense Systems product page.




